Primary Causes of Acid Rain
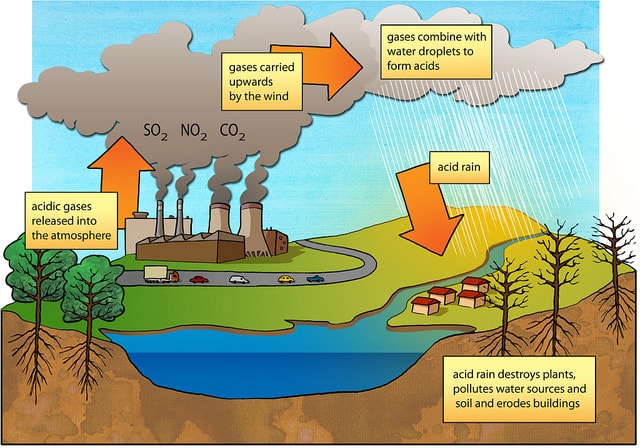
Acid rain is formed by elevated levels of sulfur and nitric acids in the atmospheres that accumulate as a result of Nitrogen oxides (NOx) and Sulfur dioxides (SO2) emissions. Acid rain is a mix of atmospheric water molecules and dry depositions of Sulfur dioxides and Nitrogen oxides emitted from industries and vehicles. When these gases chemically react with atmospheric water molecules, mild sulfuric and nitric acids are formed which fall back on land as acid rain.
In precise, acid rain is defined as any form of precipitation such as fog, sleet, snow or rain that has been made acidic by Nitrogen oxides and Sulfur dioxide pollutants in the atmosphere. Acid rain is generally caused by man-made and natural factors.
Here are the widely recognized causes of acid rain.
Man-made or Anthropogenic Causes of Acid Rain
Human activities leading to chemical gas emissions such as sulfur and nitrogen are the primary contributors to acid rain. Factories, power generations facilities, and automobiles are the chief emitters of sulfur and nitrogen gases. Use of coal for electrical power generation is the biggest contributor to gaseous emissions leading to acid rain.
Automobiles and factories also release high scores of gaseous emissions on a daily basis into the air, especially in highly industrialized areas and urban regions with large numbers of car traffic. As a result, these areas experience exceedingly high amounts of acid rain. Let’s look at the details.
1. Combustion of coal and oil
As stated earlier, the principal emissions accountable for acidic depositions in the atmosphere are oxides of nitrogen (NOx) and sulfur dioxide (SO2). Combusting coal and oil emit loads of these gases into the atmosphere. Once in the atmosphere, these compounds react with atmospheric water molecules in the presence of sunlight to form mild sulfuric and nitric acids.
Coal and oil are burned to produce energy to power machinery and are also used for heating, cooking, and lighting. The bottom line is, combustion of these substances releases the core gases responsible for the formation of acid rain.
2. Power plants and manufacturing industries
Contemporary power plants use fuel to generate energy. In the process of energy generation and combustions, sulfur dioxide and nitrogen oxide gases are released into the atmosphere. Manufacturing industries that manufacture cement, refine petroleum, process plastics, produce chemical products and pharmaceuticals or those that produce metals such as steel and aluminum release scores of NOx and SO2 gases into the atmosphere.
A much as these industries use technologies to reduce the emission of NOx and SO2 gases, the pollutants are released in small amounts but continuously for prolonged time periods eventually leading to the formation of acid rain.
3. Automobiles and other vehicles
Another main source of NOx and SO2 emissions are the fuel combustion of trucks, cars, airplanes, and buses. These automobiles release high levels of sulfur and nitrogen gaseous emissions on a daily basis into the atmosphere, mainly in highly industrialized areas and urban areas with large numbers of car traffic. Accordingly, these areas usually experience substantial amounts of acid rains.
The sulfur and nitrogen emissions can also be blown to other regions leading to acid rain formation in other areas away from the emission sources. For instance, it is believed the acid rain in Sweden is influenced by air pollution in Britain.
Natural causes of Acid Rain
Natural causes of acid rain are relatively small compared to those from anthropogenic sources as discussed above. The following are the most common natural causes of acid rain.
1. Volcanic eruptions: The main natural causal agent for acid rain is volcanic emissions. Volcanoes emit acid-producing gases, mainly sulfur, to create higher than normal amounts of acid rain or any other form of precipitation such as fog or snow to an extent of affecting vegetation cover and health of residents within the surrounding.
- Decaying vegetation, wildfires, and biological processes: Decaying vegetation, wildfires, and biological processes within the environment also generate the acid rain forming gases. Dimethyl sulfide is a typical example of a major biological contributor to sulfur-containing elements into the atmosphere.
- Lightning: Lightning strikes naturally produce nitric oxides that react with water molecules via electrical activity to produce nitric acid, thereby forming acid rain. Lightning is the major natural source of NOx.
Photo by: Siyavula